In the first post of this series, I described the basis for how we became involved in determining a method for the detection of a natural, biogenic source of toluene. In this post, I describe how the process works and provide some thoughts on how to conduct an investigation.
The backstory
- Petroleum is formed over a very long time, at moderate temperatures. As organic biological chemicals decompose they produce a distribution that is largely based on chemical thermodynamics. Not the most blog-worthy topic, but stick with me…
- In contrast, chemicals that are formed from biological processes follow a very different pathway, which is highly specific. Microbes do not produce a vast thermodynamic soup when they use organic chemicals as part of their energy-chain, they are very specific.
This is the key concept for differentiating biogenic toluene and petrogenic toluene.
When we studied results from our investigations, we found that what we suspected to be biogenic toluene was present with only a very few other chemicals; para-cymene and benzaldehyde. We believe that toluene is formed as part of a biological process that includes these chemicals. With para-cymene decomposing to toluene, itself then decomposing to benzaldehyde. The start of this entire process is likely to be terpenes. Terpenes are that forest pine smell you get when you are... in the forest.
Applying visual chromatogram comparisons
All petroleum products produce a soup of alkylated benzenes, including toluene, although there are distinct differences in the patterns. There is a large thermodynamic mixture present. So, for petroleum sources, chromatograms are filled with a variety of alkylated benzenes.
The samples of peat from our investigations presented startlingly clean chromatograms, with only a few chemicals present – toluene, para-cymene and benzaldehyde.
So, simply visually inspecting the chromatograms is a quick and easy method for determining the origin of toluene, but we wanted to go one step further.
Applying forensic ratios
Constructing ratios of toluene with benzene, ethylbenzene and xylenes is nothing new. It is carried out (with strong caution), for environmental forensics investigations. Applying a ratio of these chemicals for investigating biogenic toluene, we found that the ratio of T/BTEX is very high for natural sources, and low for petroleum sources.
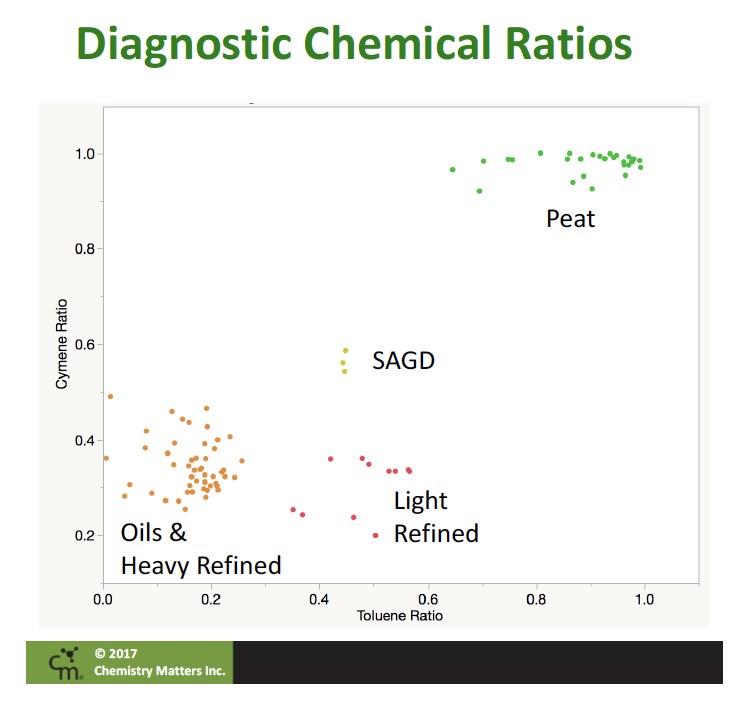
We also were able to demonstrate that the ratio of the three isomers of cymene (ortho, meta, para) could be a very useful tool. The para-cymene isomer is strongly favoured in normal decomposition processes and a thermodynamic mixture is favoured in petroleum products.
Using these ratios we could construct a graph, called a double ratio plot. These are common tools in environmental forensics, with sufficient evidence that the ratios are suitable for separating sources. When we plotted data from our investigations along with a whole range of Alberta oils and distillates, it was very clear that biogenic and petrogenic toluene sources plotted in distinct regions. A graphic from one of our presentations shows the distinctive regions of the double ratio plots showing the difference in petrogenic and biogenic toluene.
We have recently published an article that covers this topic in detail which can be found on the SETAC website for Environmental Toxicology and Chemistry (ET&C). The next few blog posts will go through the methodology of providing sufficient data to demonstrate that the toluene is natural on your site. In the next blog, I will discuss the sampling requirements needed to prove that toluene is from natural sources as this is not just a one sample and your toluene issues are over!

-1.png?width=859&name=Sample%20%231%20(2)-1.png)

.jpg?width=859&name=Archery%20photo%20(3).jpg)