I was working through a report and presentation my company had made on environmental forensics fingerprinting of polycyclic aromatic hydrocarbons (PAHs) from a spill event. I really was enjoying the project because all the data 'made sense'. Nothing makes you feel more confident about results from a big project when the science and data all make sense and everything is nice and logical. Everything but one little thing.
There was still one minor detail that didn't quite match up but it wasn't a big thing and didn't really impact the results. As we were working through the communication presentation that would be used to make the data public, I was explaining how there are natural PAHs that can be found in sediments. This, in addition to the background PAHs, found in the sediments provides a 'reference' signal that is 'naturally' present in all samples, regardless of location.
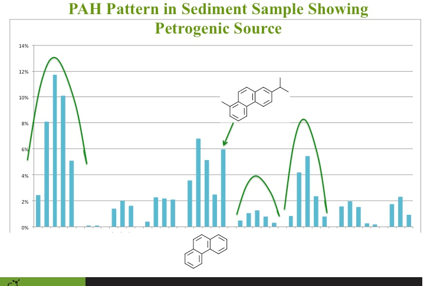 As I was going through the presentation with the client, we were looking at the PAH sediment data presented in a bar graph and one of the bars was not in the pattern it was suppose to be. If you plot PAHs and their alkylated homologues, petrogenic sources of PAHs should be 'bell' shaped (See figure). The phenanthrene pattern was bell shaped except for the last bar which was much higher then it should have been if only from a petrogenic source. On the spot, it occurred to me what this was. Retene is a natural PAH formed during the degradation of diterpenoids produced in conifers. It made perfect sense for this compound to be present at high concentrations in these sediment samples. Crisis averted... the world made sense once again. My client was happy and quite impressed what chemical fingerprinting can do for data on PAHs. If the client is happy, everyone is happy.
As I was going through the presentation with the client, we were looking at the PAH sediment data presented in a bar graph and one of the bars was not in the pattern it was suppose to be. If you plot PAHs and their alkylated homologues, petrogenic sources of PAHs should be 'bell' shaped (See figure). The phenanthrene pattern was bell shaped except for the last bar which was much higher then it should have been if only from a petrogenic source. On the spot, it occurred to me what this was. Retene is a natural PAH formed during the degradation of diterpenoids produced in conifers. It made perfect sense for this compound to be present at high concentrations in these sediment samples. Crisis averted... the world made sense once again. My client was happy and quite impressed what chemical fingerprinting can do for data on PAHs. If the client is happy, everyone is happy.


-1.png?width=859&name=Sample%20%231%20(2)-1.png)
.jpg?width=859&name=Archery%20photo%20(3).jpg)